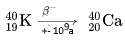Three radioactive nuclei present since the formation of the earth 4 billion years ago are at the origin of the natural radioactive series. They are mainly found in granitic rocks. Those are: $_{92}^{238}U$ Uranium 238 $_{90}^{232}Th$ Thorium 232 $_{19}^{40}K$ Potassium 40
Let's follow its successive decays:
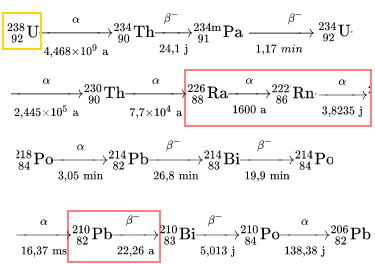
We notice: -The appearance of radium $ _{88}^{226}Ra $ with a fairly long half-life, which allowed Marie Curie to isolate it. -The formation of radon gas $ _{86}^{222}Rn $, dangerous, especially inside unventilated dwellings. -The intermediate formation of radioactive lead $ _{82}^{210}Pb $, problematic, with a rather long half-life, in the rain waters (Rn -> ... clouds - > waters)
Let's follow its successive decays:
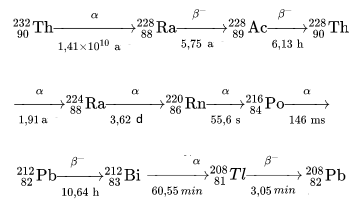
We notice: Intermediate nuclei have fairly short lifetimes!
It mainly gives rise to a $\beta^-$ decay!