





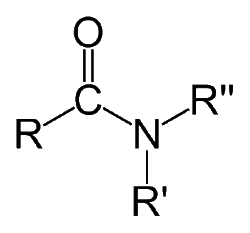
 $R',R''$ = $H$ or alkyle(N- substituted amide)
$R',R''$ = $H$ or alkyle(N- substituted amide)

- The hydrogen bonds between amide molecules explain their high melting and boiling temperatures:
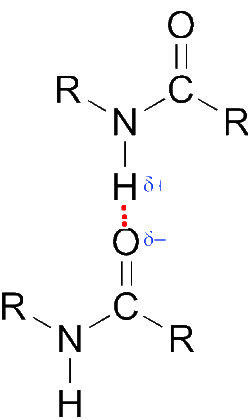 Methanamide is liquid, while ethanamide is already solid at normal temperature.
Methanamide is liquid, while ethanamide is already solid at normal temperature.
- The hydrogen bonds between amide molecules and water molecules explain the solubility of amides in water:
 From heptanamide, however, the hydrophobic character of the hydrocarbon chain wins out and the solubility becomes weak and then nil.
From heptanamide, however, the hydrophobic character of the hydrocarbon chain wins out and the solubility becomes weak and then nil.
1) Reaction with ammonium carbonate to form ammonium carboxylate: $ 2 R-COOH + (NH_4)_2CO_3 \rightarrow 2 R-COO^-NH_4^+ + 2CO_2 + 2 H_2O $ then heating to form the amide by dehydration: 2) $ 2 R-COO^-NH_4^+ \rightarrow 2 R-CO(NH_2) + 2H_2O $
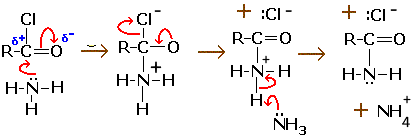
Violent reaction with ammonia to form amide and ammonium chloride:
$ 2 R-COCl + 2NH_3 \rightarrow R-CO(NH_2) + NH_4^+Cl^- $
The mechanism proceeds by nucleophilic attack of ammonia on the carbon of the acid chloride function:

Amides (b) are intermediate between nitriles (a) and ammonium carboxylates (c) - (1) Dehydration by $ P_2O_5 $ - (2) Hydration with diluted $ HCl$ - (3) Hot dehydration - (4) Hydrolysis -with dilute $ HCl$ gives the ammonium carboxylate (c) which is then converted (5) to carboxylic acid (d) - with dilute $ NaOH $ gives the ammonium carboxylate (c) which is then converted (6) into carboxylate ion (e)
The mesomerism:
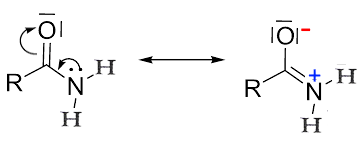 produces a positive partial charge on the nitrogen. This charge retains the doublet of nitrogen, which greatly reduces the basicity of the amides.
produces a positive partial charge on the nitrogen. This charge retains the doublet of nitrogen, which greatly reduces the basicity of the amides.
Name the final substances obtained from the reagents: - Propanoyl chloride and dimethylamine →
- Methanamide and dilute hydrochloric acid →
- N -propyl- N -methylethanamide and dilute sodium hydroxide →