





In this case, the doublet participates in the mesomerism of the cycle and generates negative charges in the ortho and para in the in the contributive mesomeric forms: Example: Phenol
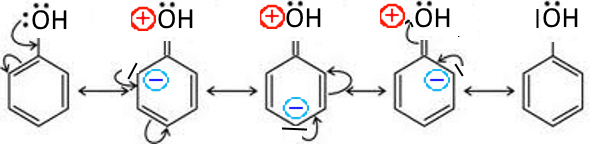
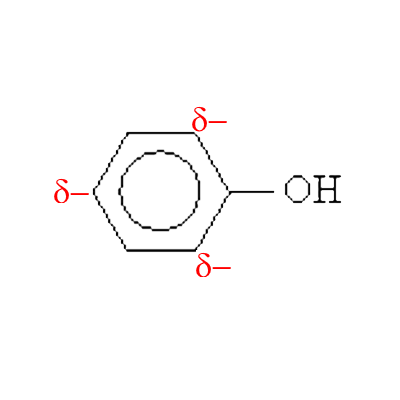
Phenol thus carries partial negative charges in ortho and para positions.
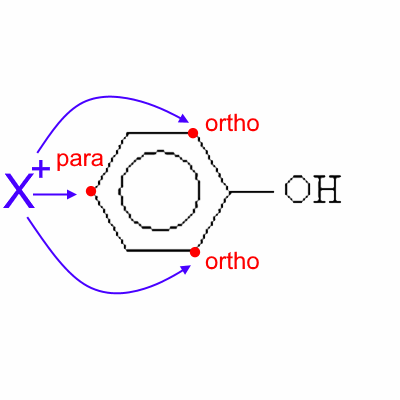
 An electrophilic reagent X+ is therefore preferably attacking phenol in ortho and para positions.
An electrophilic reagent X+ is therefore preferably attacking phenol in ortho and para positions.
In this case, a doublet of benzene tends to prolong the mesomerism towards this atom and shows positive charges in ortho and para in the contributive mesomeric forms: Example: Benzoic acid
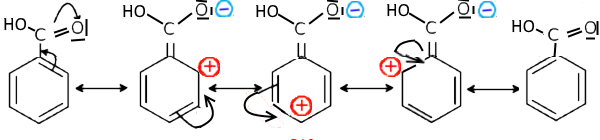
The benzoic acid thus carries partial positive charges in ortho and para positions.
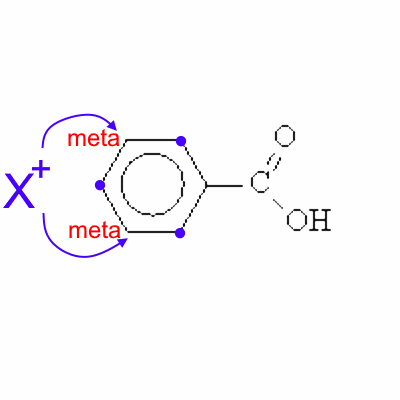
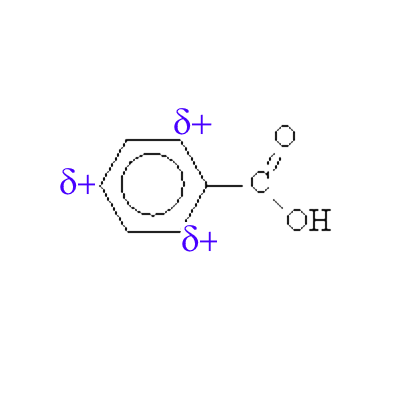 An electrophilic reagent X+ will therefore not attack at these positions, but preferably in meta .
An electrophilic reagent X+ will therefore not attack at these positions, but preferably in meta .
 → Exercises
→ Exercises