







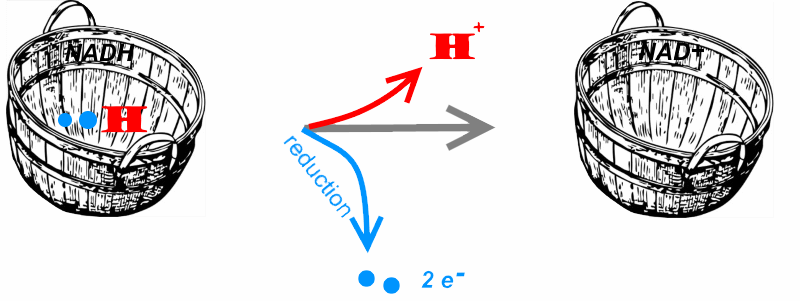
 The $NADH$ can be compared to a basket filled with a hydrogen ion and two electrons.
It can transmit these electrons so act as reducer .
The $NAD^+$ is comparable to the empty basket.
It can recapture both electrons and hydrogen ion (dehydrogenate!), so act as oxidant.
The $NADH$ can be compared to a basket filled with a hydrogen ion and two electrons.
It can transmit these electrons so act as reducer .
The $NAD^+$ is comparable to the empty basket.
It can recapture both electrons and hydrogen ion (dehydrogenate!), so act as oxidant.
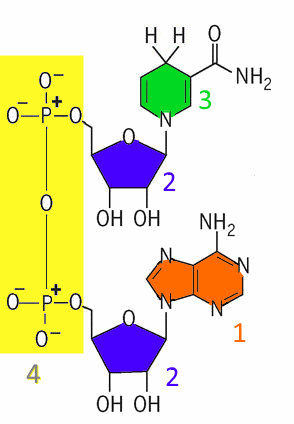
1 : adenine 2 : ribose 3 : nicotinamide 4 : diphosphate group Hence the name: NicotinamideAdenineDinucleotide
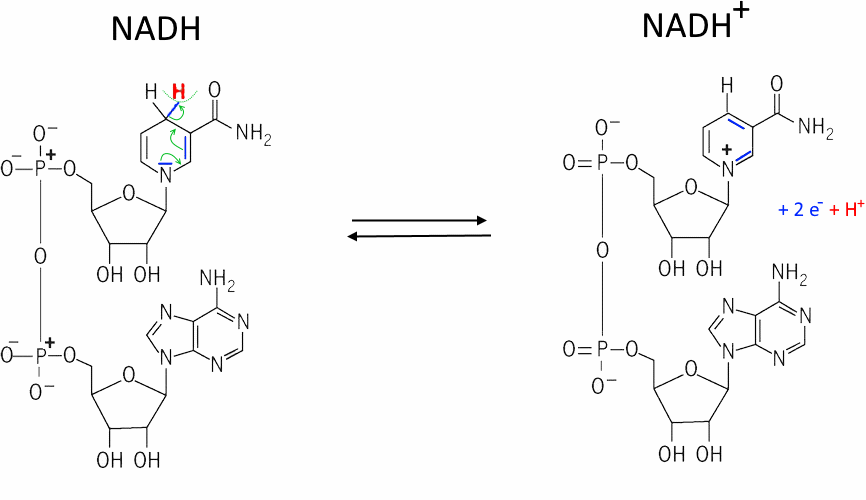
The exchange $H^+$ and 2 $e^-$ happens in the pyrimidine nucleus of nicotinamide.
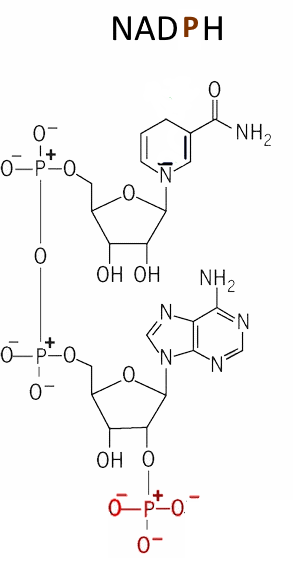
The exchange $H^+$ and 2 $e^- $ is done as for the $NADH $ While $NAD^+ $ is more active in oxidations eg → glycogenolysis , $ NADPH $ is more active in reductions e.g. → photosynthesis