





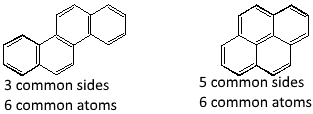
Ortho-condensed systems: Polycyclic systems in which two adjacent rings have two, and only two atoms in common ( n common sides and 2n common atoms). Ortho and peri-condensed systems: Polycyclic systems wherein one ring contains two, and only two adjacent atoms in common with each of the two (or more) cycles of a continuous series of ortho-condensed rings ( n common sides and less than 2n atoms in common).
See here a non exhaustive list of → Polycyclic hydrocarbon compounds. See alsom here a non exhaustive list of → Polycyclic heterocyclic compounds
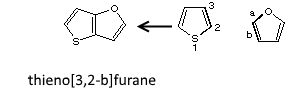
Polycyclic systems with the maximum number of non-cumulative double bonds (and not having a trivial or semi-trivial name) are named by choosing a component with a trivial or semi-trivial name as a base component and by coding the other components by prefixes. So a heterocycle has priority over a carbocycle. Precedence in the heterocycles and carbocycles results of the order of the prior lists.
Prefixes designing a fixed component are formed, in general, by replacing the "e" terminal of the cycle with an "o": pyrrolo, pyrimido, ... Some prefixes are contracted: benzo (acetamido) naphtho, anthra, phenanthro, cyclobuta, cyclopenta, thieno, ...
The base component bonds are distinguished by letters "a, b, c, ..." the other component bonds are numbered "1, 2, ..." The indices are placed brackets.
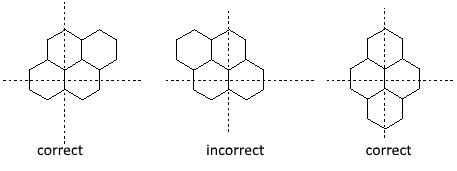
Orientation of a polycyclic system : - Maximum horizontal cycles in a row - Maximum number of cycles above and at the right of the "horizontal line". - If two (or more) orientations are possible, we choose the one with the less cycles possible in the bottom left section.
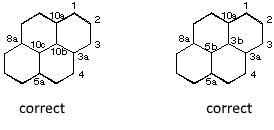
Numbering of a polycyclic system: - Numbering in the clockwise direction beginning by the first atom that is not engaged in a ring fusion and which is the at the left in the highest cycle - Common atoms to one or more rings are omitted (except hetero atoms) - Common atoms to one or more rings are designated by adding the Roman letters (a, b, ...) to the number of the previous position. - Interior atoms have the highest numbers (numbering in the clockwise direction ).
A complete IUPAC version of this matter is found → here