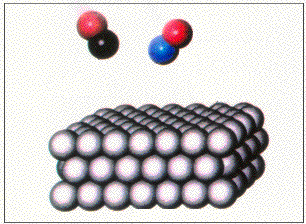
The heterogeneous catalysis involves the use of a catalyst in a different phase from the reactants.
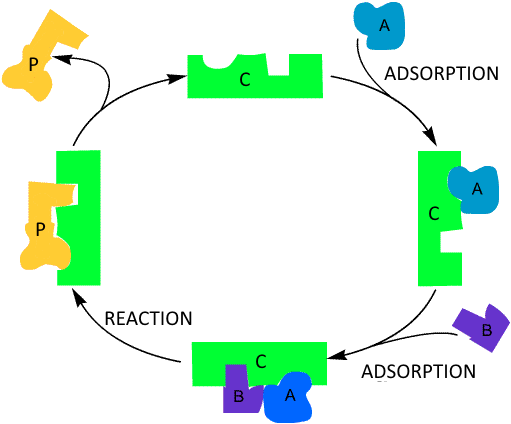
Often reagents (here $A$ and $B$) are adsorbed on a solid catalyst ($C$) and there they combine to form the product(s) ($P$) that is (are) liberated later:
In catalytic converters for cars a reaction (among others) is the conversion of the toxic gases nitrous oxide and carbon monoxide into carbon dioxide and nitrogen: $2\;NO$ $+$ $2\;CO$ $\longrightarrow$ $2\;CO_2$ $+$ $N_2$ This reaction is accelerated on a solid catalyst, the rhodium metal. The mechanism seems to be the following: