Aldehydes and alcohols react (in acid fast) to form hemiacetals:
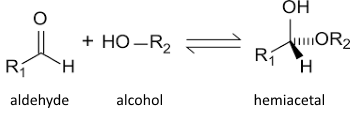
Propose a reaction mechanism!
?
Hemiacetals are often unstable substances, except in the case of sugars!
The cyclization happens by hemiacetalisation in water:
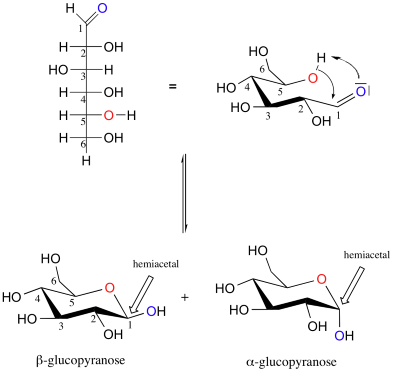
In the water, there is at equilibrium: - Less than $ 1 \%$ linear form - $ \approx 63 \% $ of $ \beta $ form - $ \approx 37 \% $ of $ \alpha $ form
This spendid video is due to Heisenberg12051901
Representations of cyclic forms can be simplified as follows:
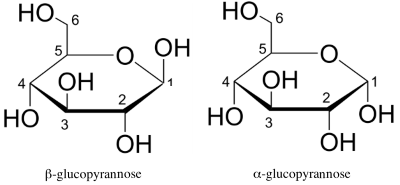
- The lower percentage of the ("anomeric") $\alpha$ enantiomer in equilibrium is explained by the higher reciprocal steric hindrance $OH$ positioned in 1 and 2, on the same side of the cycle! - The cyclization of (L)-enantiomers of glucose lead to the following:
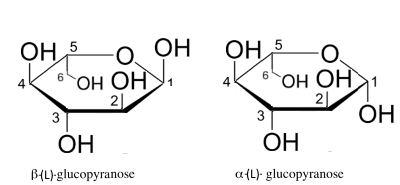
- Note that in the form (D) $OH$ that were right in linear Fischer form are now found at the bottom of the cycle, in the form (L) at the top and vice versa. - Note again that in the form (D) $ CH_2OH$ is found at the top of the cycle, in the form (L) down. - Note again that in both forms the anomeric $OH$ of carbon 1 is always down for the $\alpha$ form , top for the $\beta$ form.
Give the Haworth projection - The $ \beta- (D) $ - altropyranose - The $ \alpha (L) $ - galactopyranose
?
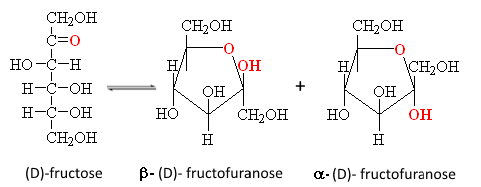
For these cyclizations apply the same rules as for aldoses, hydrogen of the aldehyde function is replaced by the group $ CH_2OH $