







Identify the base peak and the molecular peak of the following mass spectrum. What is the molar mass of the substance?
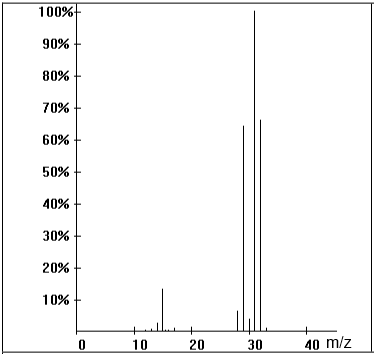
$\frac{m}{z}$ $=$ $31$ $\Rightarrow $: base peak $\frac{m}{z}$ $=$ $32$ $\Rightarrow $ molecular peak $ M$ $=$ $32 \frac{g}{mol}$
What causes the peak $\frac{m}{z}$ $=$ $32$ ?
This peak is probably due to the isotope $^{13}C$ present in the substance.
What causes the peak $\frac{m}{z}$ $=$ $15$ ?
This peak is probably due to the ion $CH_3^+$
Do you see a very specific peak?
The peak $\frac{m}{z}=31 $ is due to the ion $CH_2OH^+$
What substance is this?
Methanol $CH_3OH$