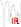





Search:
Infrared light accessible to our instrumentation covers wavelengths ranging from $2.5\;-\;15\mu m $. Today we are rather accustomed to use the wave number:
Wave number($cm^{-1}$) = 10000/wavelength($\mu m$)
The accessible spectrum reaches from $4000 - 666 cm^{-1}$. A sample of the substance to be analyzed is subjected successively to the entire spectrum and the percentage of transmitted light depending on the infrared wave number is measured.
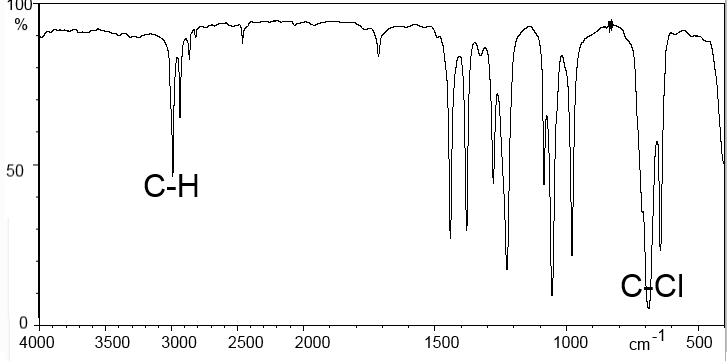
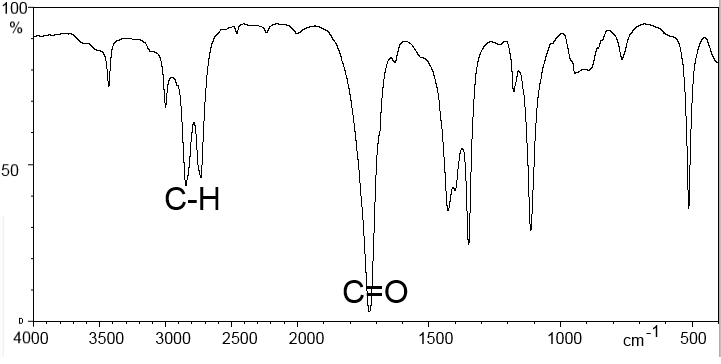
We note that groups of atoms, such as $C = C$, $C = O$, $C-H$ etc absorb IR light in specific locations of the spectrum. This position may vary slightly depending on the environment of the group.
You can find more details here Hence the infrared spectra:
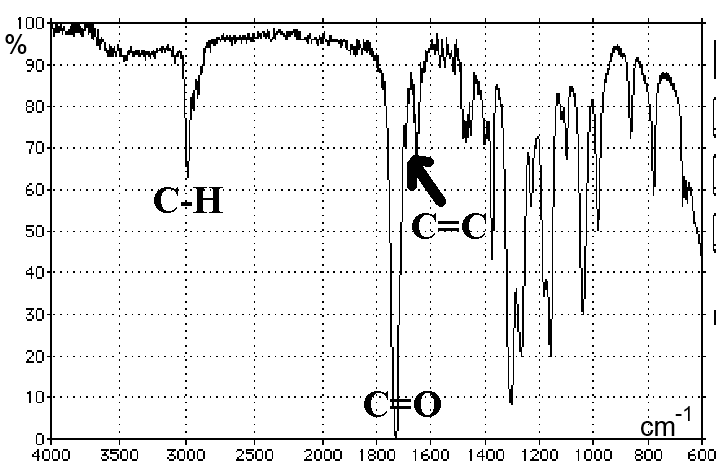
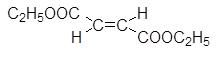
Typically we find on the abscissa the wave number (in $cm^{-1}$), on the ordinate the transmittance, ie the percentage of IR radiation transmitted. The peaks thus correspond to a stronger or weaker absorption of IR rays.
Cyclopentane:
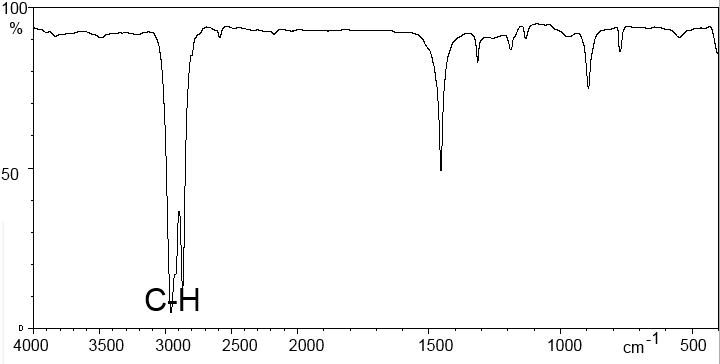
Methanol
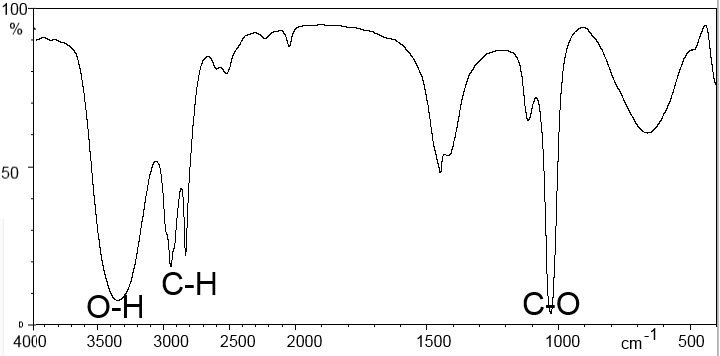
Methanoic acid:
Ethanal:
1,1-Dichloroethane: