





The oxidation products are acetaldehyde and ethanoic acid. The ethanol, acetaldehyde and ethanoic acid have the same oxidation number of all their corresponding identical atoms, except for $C$:
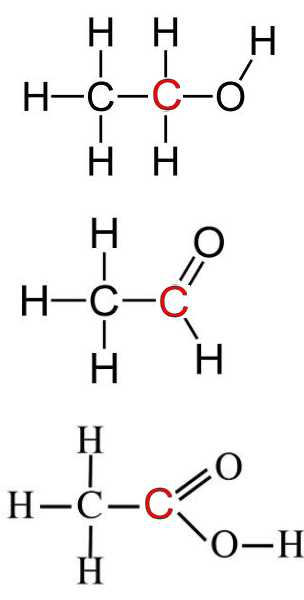
Reminder:Oxidation numbers (n.o.) are determined by attributing to the more electronegative atoms all electrons of the bond, and the dummy load thus obtained is the oxidation number.
Primary alcohol $\Rightarrow$ aldehyde $RCH_2OH$ $-$ $2e^-$ $\rightarrow$ $RCHO+2H^+$ Aldehyde $\Rightarrow$ carboxylic acid $RCHO$ $-$ $2e^-$ $+$ $H_2O$ $\rightarrow$ $RCOOH+2H^+$
The only oxidation product is acetone ( propanone ). Isopropanol and acetone have the same oxidation number of all their corresponding identical atoms, except for $C$
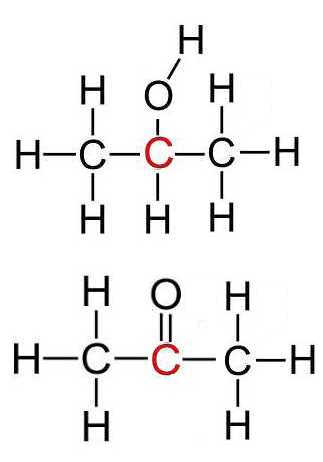
Secondary alcohol $\Rightarrow$ ketone $R_1R_2CHOH$ $-$ $2e^-$ $\rightarrow$ $R_1R_2C=O$ $+$ $2H^+$
Under stringent conditions, the oxidation is carried out breaking the molecule, an controlled oxidation is impossible.