





The initiator is a peroxide, often the $-COOC-$ group, that dissociates under the effect of heat:
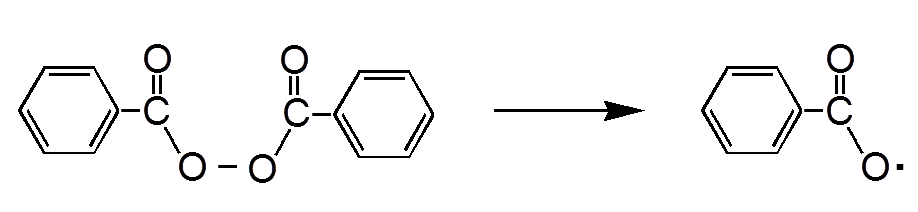
The dissociation of benzoyl peroxide (here) is homolytic ("equals"). There are formed radicals , ie chemical species (uncharged) with an unpaired electron.
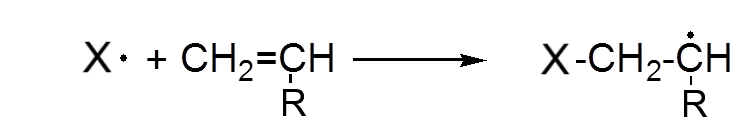
A radical attacks the π bond of an alkene and dissociates it to form a new radical.
The new radical in turn attacks a second alkene etc ...
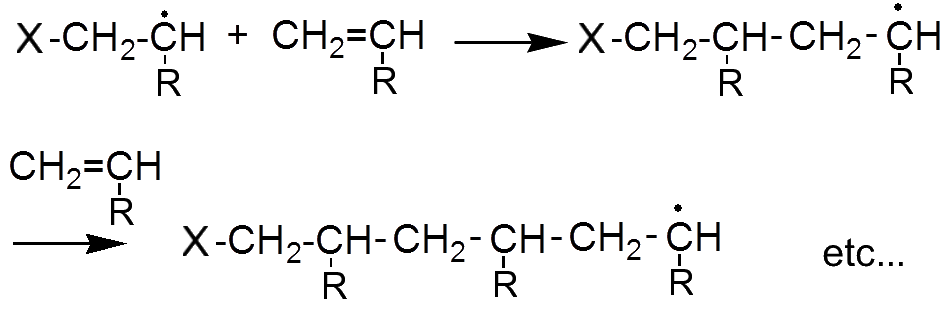
The reaction is complete when two radicals meet.

For the same polymerization, the polymer molecules can have different chain lengths. Indeed, a polymer is therefore not a chemical substance (identical molecules). By reducing the amount of initiator, we will produce less radicals at one time and the length of the final chain will be larger, because these radicals are less likely to meet.