





The bond angle $COH$ and the strong difference of electronegativities is why the alcohol is highly polar.
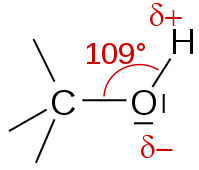
Water is also highly polar. The molecules of water and alcohol combine to form hydrogen bridges. This explains the very good solubility of small chain alcohols in the water.
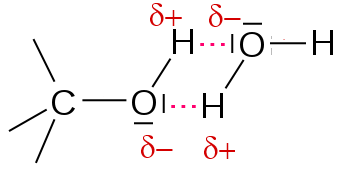
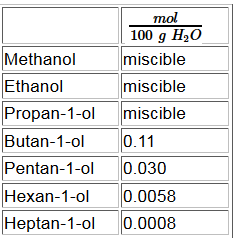
Once the chain of carbon atoms grows longer, the hydrophobicity of the (non-polar, "afraid of water") hydrocarbon chain outweighs the hydrophilic (polar, "water-loving") caracter of the hydroxyl group .
H bridges provide a strong mutual attraction of alcohols.
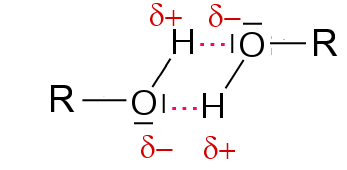
Their volatility is much smaller than that of alkanes that are held together only by their feeble strength of van der Waals forces.
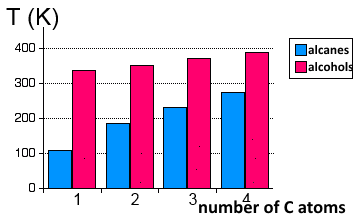
Boiling points compared of linear alkanes and alcohols in degrees Kelvin. .
We see that as the C atom chain grows longer, the hydrophobic nature of the hydrocarbon chain outweighs the hydrophilic nature of the hydroxyl group and the volatily of alcohols approaches that of alkanes of comparable molar masses.