





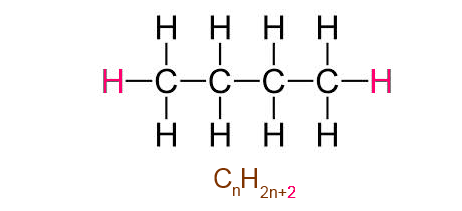
The number of hydrogen atoms is the double of the number of carbon atoms + 2
Here are the changes that may occur comparing to linear alkanes:
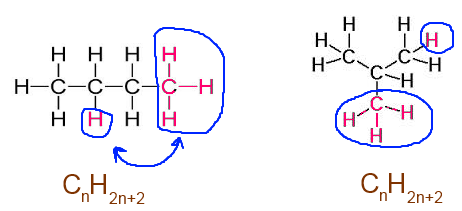
Ramification: No modification
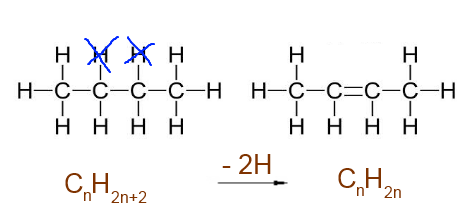
Double bond C=C : -2 H atoms
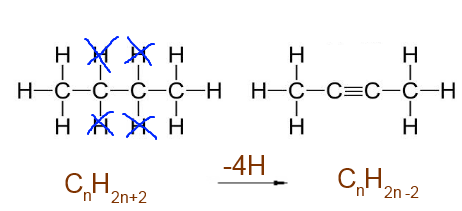
Triple bond C$\equiv$C : -4 H atoms
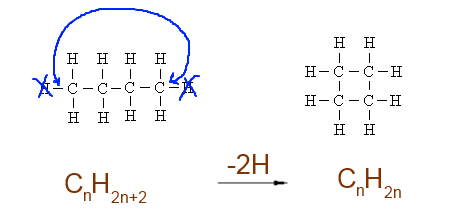
Cycle : -2 H atoms
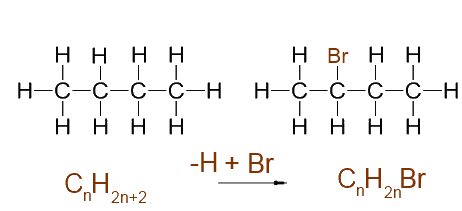
One added halogen : - 1 H atom+ 1 halogen atom
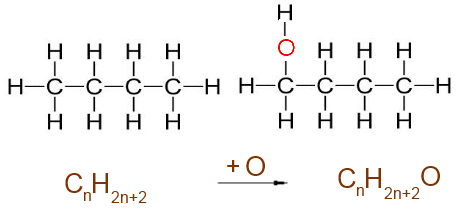
Oxygen interposed between C and H : + 1 O atom
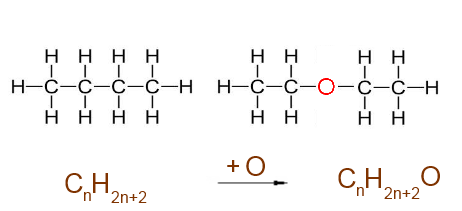
Oxygen interposed between C and C : + 1 O atom.

Double bond C=O : -2 H atoms + 1 O atom
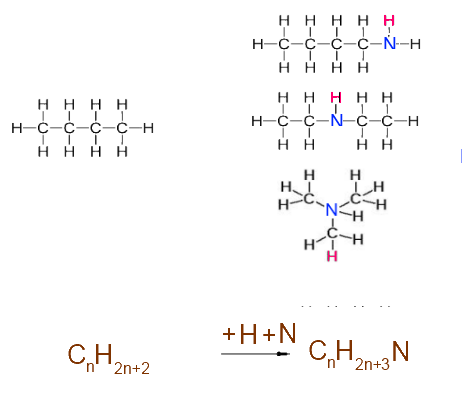
Nitrogen interposed : +1 H atom + 1 N atom
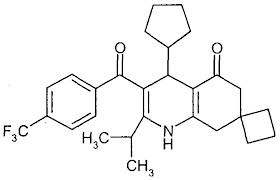 The molecule has 28 C atoms, The corresponding linear alkane would have the formula $C_{28}H_{58}$
5 cycles:→ -10H
5 C=C:→ -10H
2 C=O→ -4H+2O
1 N:→ +1H+N
3 F:→ -3H+3F
..so the molecular formula is:
$C_{28}H_{32}O_2NF_3$
The molecule has 28 C atoms, The corresponding linear alkane would have the formula $C_{28}H_{58}$
5 cycles:→ -10H
5 C=C:→ -10H
2 C=O→ -4H+2O
1 N:→ +1H+N
3 F:→ -3H+3F
..so the molecular formula is:
$C_{28}H_{32}O_2NF_3$