





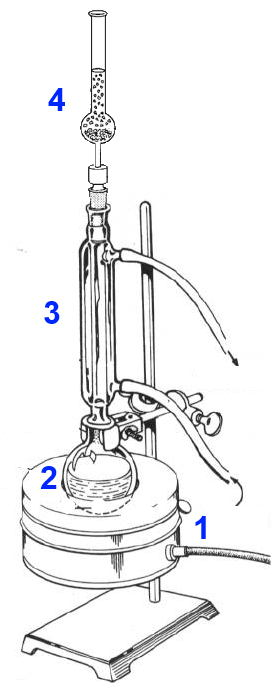
In the flask 2 are successively added - $25\; ml$ of ethanol $CH_3CH_2OH$ - $25\; ml$ of pure ethanoic acid (acetic acid $CH_3COOH$ ) - ( slowly, cooling and stirring ) 15 mL of concentrated sulfuric acid $H_2SO_4$ The condenser 3 is fixed and the calcium chloride tube 4 Water enters in the condenser from below. By regulating the heating mantle, the mixture is made to boil gently for 10 minutes. The vapor condenses in the condenser and flows back into the flask. The $CaCl_2$ tube prevents the entry of moisture.
$CH_3COOH$ $+$ $CH_3CH_2OH$ $\rightleftarrows$ $CH_3COOCH_2CH_3$ $+$ $H_2O$
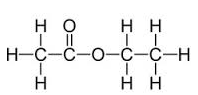
Ethyl acetate
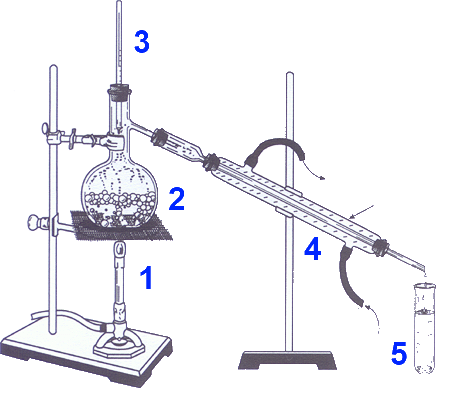
In the flask introduce the products obtained by reflux. Heat on metal gauze using a Bunsen burner 1 Be limited to a vapor temperature of $100^oC$ with the thermometer 3 Condense the refrigerant vapor in the condenser 4 Collect the liquid products in 5
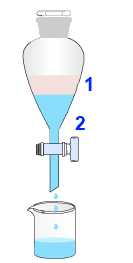
Introduce into the funnel - the products obtained by distillation 1 - $12.5 \;mL$ $30 $% $Na_2CO_3$ solution 2 . Shake allowing the opening of the stopper . $CO_2$ emerges as a result of the reaction: $2CH_3COOH$ $+$ $Na_2CO_3$ $\rightarrow$ $ 2CH_3COONa$ $+$ $H_2O+CO_2$ Sodium acetate is soluble in water and enters phase 2. Decant 2 and keep the pure ester 1

Ethyl acetate is a toxic, volatile and highly flammable colorless liquid with a fruity odor.