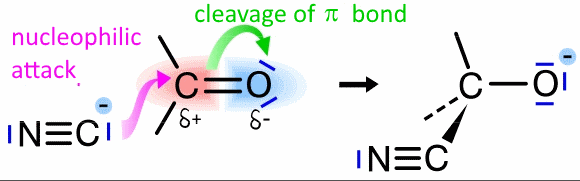
Click on the image!
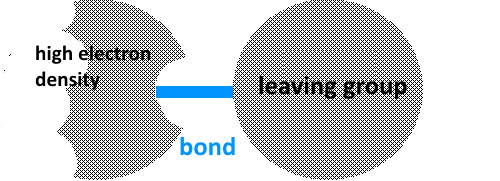
A leaving group or nucleofuge is part of a molecule that carries away the bonding electron pair by which it was attached to the molecule thus completing its octet. A high electron density center is part of a molecule which pushes the electron pair of the leaving group to enable it to carry away a pair of bonding electrons. The quality of the leaving group (importance of nucleofugality) depends on several factors: 1) The charge: Nucleofugal neutral leaving groups depart easier than leaving anions. Indeed, carrying away a pair of electrons, these groups often leave behind a positive charge and have thus more difficulty to start as they are negatively charged. By descending nucleofuge quality , we find for example: $H_2O$ water $OH^-$ hydroxide ion 2) The size of the atom on which the leaving group is attached. The atoms that are bigger allow better the movement of electrons in their electron cloud (they are more polarizable)! By descending nucleofuge quality , we find for example: $I^-$ iodide ion $Br^-$ bromide ion $Cl^-$ chloride ion $F^-$ fluoride ion We see that the nucleofuge quality decreases from top to bottom in a family in the periodic table: $I$ $\gt$ $Br$ $\gt$ $Cl\gt$ $F$ 3) The solvent. An acid solvent can for example facilitate the departure of $OH$ by activating this group so that it may leave as neutral $H_2O$ :
Click on the image!
Some examples from the site of Athénée de Luxembourg → here
: