An indicator is a couple of weak acid/weak base which have both different colors.
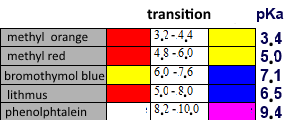
Bromothymol blue is a couple consisting of a weak yellow acid and a blue corresponding base.

The cyclinder A contains mainly acid, B cylinder mainly base and cyclinder C as much base than acid
Bromothymol blue is a buffer $HB/B$ with $pKa$ $=$ $7.10$ that obeys the relation: $pH$ $=$ $pKa+log(\frac{[B]}{[HB]})$ We have: $\frac{[B]}{[HB]}$ $=$ $10^{pH-7.10}$ so, for $pH$ $\gt$ $7.10+1$ there will be 10 times more of the blue species than of the yellow one and the indicateur will have a blue colour, for $pH$ $\lt$ $7.10-1$ there will be 10 times more of the yellow species than of the blue one and th indicateur will have a yellow colour.
Indicators and $pH$ domains: $pH \gt pKa +1$ : Colour of the base $pKa-1 \lt pH \lt pKa +1$ : intermediate colour (turn) $pKa-1 \lt pH$ : Colour of the acid
The transition range of an indicator is approximately the interval $[pKa -1, pKa +1]$.