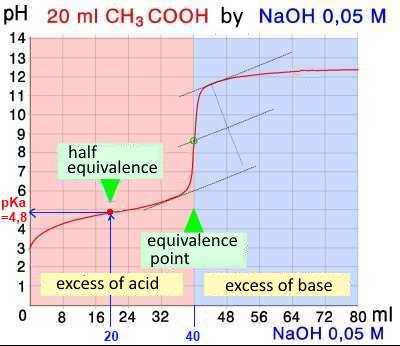
The half equivalence point of a titration is the point where the number of moles of added titrant is just half of the number of moles of the titrated reagent on departure.
Reagent 1 (weak) is titrated by reagent 2 (strong): At the half equivalence point half of the (strong) titrating reagent 2 has reacted to form the base or the acid corresponding to the weak titrated reagent 1, it results in a buffer with equal numbers of moles of acid and base: $pH$ $=$ $pKa_1$ $+$ $log(\frac{n_{base1}}{n_{acide1}})$ $pH$ $=$ $pKa_1$ $+$ $log\;1$ $pH$ $=$ $pKa_1$
The $pH$ at the point of half equivalence is equal to the $pKa$ of the weak titrated reagent