





By its dissociation,
$B$ $+$ $H_2O$ $\rightleftarrows$ $ OH^-$ $+$ $HB$
a base, however weak, usually brings more hydroxide ions in water that autoprotolysis of water itself.
Let's call $n_{B}$ the number of moles of base $B$ introduced into the water before it is dissociated.
If $V$ is the final volume, the initial(formal) molarity is written:
$c_{B}$ $ = $ $\frac {n_{B}}{V}$
Let's call $x$ call the number of moles of hydroxide $OH^-$ present in the solution when the dissociation equilibrium is reached.
Then we have:
$OH^-$ $=$ $ \frac{x}{V}$
and
$pOH $ $=$ $ -log(\frac{x}{V})$
Let's calculate $x$:
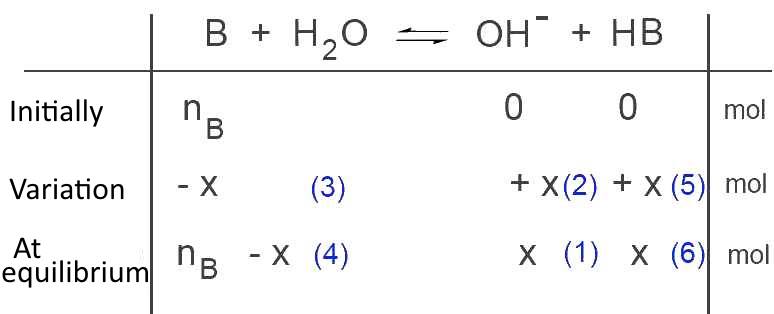 We find in order:
(1) $x$ moles $OH^-$ at equilibrium
(2) $x$ moles $OH^-$ prodiuced
(3) $x$ moles $B$ disappeared
(4) $n_B-x$ moles $B$ at equilibrium
(5) $x$ moles $HB$ prodiuced
(6) $x$ moles $HB$ at equilibrium
The molarities are at equilibrium:
$[OH^-]$ $=$ $\frac{x}{V}$
$[B]$ $=$ $\frac{n_B-x}{V}$
$[HB]$ $=$ $\frac{x}{V}$
Introducing in the expression of the basicity constant $K_b$:
$K_b$ $=$ $\frac{[OH^-][HB]}{[B]}$ =
$\frac{\frac{x}{V}\frac{x}{V}}{\frac{n_B-x}{V}}$ =
$\frac{\frac{x}{V}\frac{x}{V}}{\frac{n_B}{V}-\frac{x}{V}}$ =
$\frac{[OH^-]^2}{c_B-[OH^-]}$
$[OH^-]^2$ $+$ $K_b[OH^-]$ $-$ $K_bc_B$ $=$ $0$
Then, posing $z=[OH^-]$:
We find in order:
(1) $x$ moles $OH^-$ at equilibrium
(2) $x$ moles $OH^-$ prodiuced
(3) $x$ moles $B$ disappeared
(4) $n_B-x$ moles $B$ at equilibrium
(5) $x$ moles $HB$ prodiuced
(6) $x$ moles $HB$ at equilibrium
The molarities are at equilibrium:
$[OH^-]$ $=$ $\frac{x}{V}$
$[B]$ $=$ $\frac{n_B-x}{V}$
$[HB]$ $=$ $\frac{x}{V}$
Introducing in the expression of the basicity constant $K_b$:
$K_b$ $=$ $\frac{[OH^-][HB]}{[B]}$ =
$\frac{\frac{x}{V}\frac{x}{V}}{\frac{n_B-x}{V}}$ =
$\frac{\frac{x}{V}\frac{x}{V}}{\frac{n_B}{V}-\frac{x}{V}}$ =
$\frac{[OH^-]^2}{c_B-[OH^-]}$
$[OH^-]^2$ $+$ $K_b[OH^-]$ $-$ $K_bc_B$ $=$ $0$
Then, posing $z=[OH^-]$:
To determine the pH of a diluted solution of weak base: - we calculate a reasonable solution of the equation: $z^2+K_b\cdot z-K_b c_B=0$ - we calculate $pOH$ $=$ $-log\;z$ - we find: $pH $ $=$ $ 14-pOH$ $=$ $14$ $+$ $log\;z$
For example: Given a weak base $0.10\frac{mol}{L}$ solution with $K_b$ $=$ $4.00\cdot 10^{-2}$: The equation $z^2$ $+$ $4.0\cdot 10^{-2}\cdot z$ $-$ $4.0\cdot 10^{-2}\cdot 0.10$ $=$ $0$ provides the positive root: $z$ $=$ $8.3\cdot 10^{-3}$ and so: $pH$ $=$ $14$ $+$ $log\; z$ $ =$ $12$