





- The volume of the mother solution that we want to dissolve in the given volume is calculated.
For example, starting with one molar hydrochloric acid ($ HCl_{conc } $) to prepare a $250 \;ml$ of a $0.04$ molar solution ($HCl_{dil}$), there will be needed:
$n$ $=$ $[HCl_{dil}]\cdot V $ $=$ $ 0.04\cdot 0.25$ $ = $ $0.01 \;mol\; HCl$, ie a volume:
$v$ $=$ $\frac{n}{[HCl_{conc}]}$ $=$ $\frac{0,01}{1} $ $=$ $ 0.01 \;L$ $ =$ $ 10\; ml \;HCl_{conc}$
- Use a pipette:
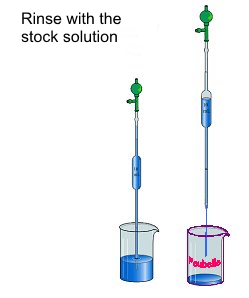
 - Rinse the pipette:
- Rinse the pipette:
 - The required volume is taken:
- The required volume is taken:
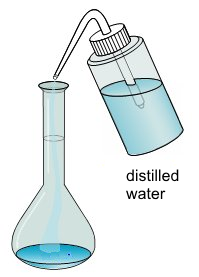
 - Distilled water is filled in:
- Distilled water is filled in:
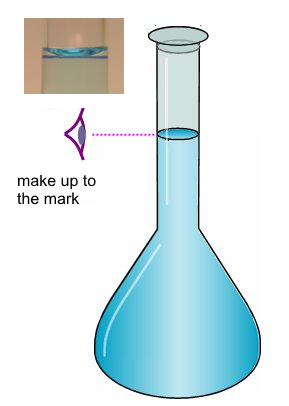
 - The calibration mark is targeted:
- The calibration mark is targeted:
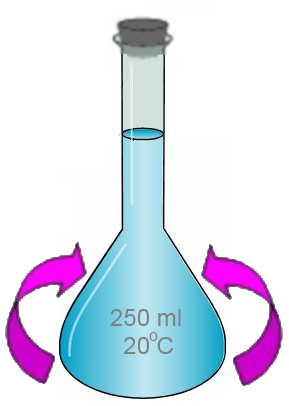
 - The flask is capped and stirred:
- The flask is capped and stirred:
 - The standard solution (the molarity is known) is ready to use!
- The standard solution (the molarity is known) is ready to use!
Images taken from: → Lycée Clemenceau de Reims