







Search:

Potassium chloride
|
1. Perchloric acid |
|
$K^+$ $+$ $ClO_4^-$ $\longrightarrow$ $ KClO_4$ - White cristalline precipitate, - slightly soluble in acids, - relatively soluble when heated |
|
2. Tartaric acid |
|
$K^+$ $+$ $HOOCCHOHCHOHCOOH $ $\longrightarrow$ $ H^+$ $+$ $HOOCCHOHCHOHCOOK$ - White cristalline precipitate, - The reaction is favoured by . by rubbing with a rod . by addition of sodium acetate . by addition of alcohol |
|
3. Picric acid or 2,4,6-trinitrophénol |
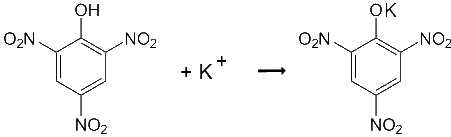
- yellow precipitate - The reaction is favoured by rubbing! - Picric acid may advantageously be replaced by dipicrylamine or hexanitrodiphenylamine |
Reactions in the absence or only applicable after removal of ammonium (see)
|
a) Sodium hexanitrocobaltate $Na_3[Co^{III}(NO_2)_6]$ |
|
$2K^+$ $+$ $Na[Co^{III}(NO_2)_6]^{3-}$ $\longrightarrow$ $ K_2Na[Co^{III}(NO_2)_6]$ - yellow precipitate - Sodium and potassium hexacobaltate, "Fischer salt" - The reaction is promoted . by heat . by the addition of alcohol! |
|
b) Chloroplatinic acid $(NH_4)_2[PtCl_6]$ |
|
$K^+$ $+[PtCl_6^{2-}]$ $\longrightarrow$ $ K_2[PtCl_6]$ - yellow precipitate |
|
Sodium tetraphenylborate "KALIGNOST" |
|
- There is formation of a white precipitate of $K[B(C_6H_5)_4] $ - This reagent in aqueous 0.1 M solution can reveal upto 4 μg of $K^+$ in 100 mL of solution. - Interfere ammonium and alkaline earth cations! |