





A thermodynamic system is a portion of matter which is subject to transformations. The (outside) environment is everything around the system.
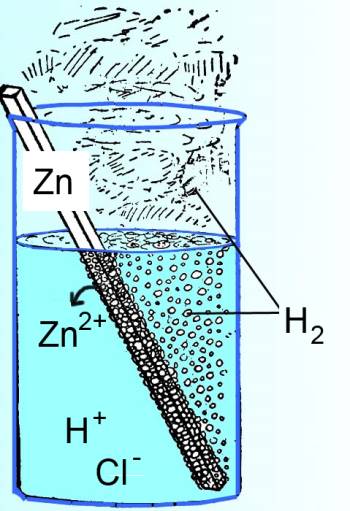
In this case, the sytem are the reagents and the products (drawn in black), the outside environment is the solvant, the container and the air on the outside (drawn in blue).
A system is isolated (closed), when an exchange with the outside environment is not possible. A system which is not isolated exchanges heat and (or) work with the environment.
In the case illustrated above, the system is not isolated: - the water is heated up by the chemical reaction: The environment receives heat from the system. - the water and the air are "pushed aside" by the hydrogen which the reaction produces: The environment receives work from the system.
Conventionally, - the plus sign (+) is given to what the system receives, - the minus sign (-) to what the system gives away.
In the case illustrated above, heat and work are negative.
The state of a system is described by a single value of its state parameters: - Pressure $P$ - Temperature $T$ - Volume $V$ - Quantities (numbers of moles) $n_i$ of all present species
2 moles of hydrogen are mixed with 1 mole of oxygen under normal conditions (n.t.p.). The reaction producing water is started. The water is collected at $4^oC$: Initial state: $P_1=1 atm$ $T_1=273,15 K$ $V_1=3\cdot 22,4L$ $n_{H_2}=2$ $n_{O_2}=1$ $n_{H_2O}=0$ Final state: $P_2=1 atm$ $T_2=277,15 K$ $V_2=36mL$$n_{H_2}=0$ $n_{O_2}=0$ $n_{H_2O}=2$