







Calculate the $pH$ of a $0.200\;M$ solution of $H_3C_6H_5O_7$
(citric acid, $K_a$ $=$ $3.5\cdot 10^{-4}$)
Equilibrium of hydrolysis:
$H_3C_6H_5O_7$ $+$ $H_2O$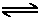 $H_3O^+$ $+$ $H_2C_6H_5O_7^-$
$H_3O^+$ $+$ $H_2C_6H_5O_7^-$
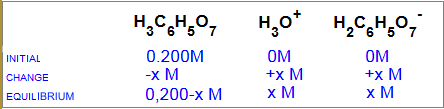
$K_a$ $=$ $3.5 \cdot 10^{-4}$ $=$ $\frac{x^2 }{ 0,0-x}$
Solving for the unknown $x$ from this equation : $x$ $ =$ $8.4 \cdot 10^{-3}\;M$ $=$ $[H_3O^+]$. How do you calculate finally the $pH$ of this solution?
a) find the $pH$ by the formula $pH$ $=$ $- log (K_a)$ b) find the $K_a$ by substituting this value of $[H_3O^+]$ in the expression of $K_a$ c) find the $pH$ by the formula $pH$ $=$ $- log [H_3O^+]$ $=$ $-log x$
The answer c) is correct, $pH =$ $-log x$ $=$ $2.08$