





$Au^{3+}+3e^-$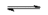 $Au$
$Hg^{2+}+2e^-$
$Au$
$Hg^{2+}+2e^-$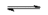 $Hg$
$Ag^{+}+e^-$
$Hg$
$Ag^{+}+e^-$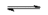 $Ag$
$Cu^{2+}+2e^-$
$Ag$
$Cu^{2+}+2e^-$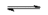 $Cu$
$2H^{+}+2e^-$
$Cu$
$2H^{+}+2e^-$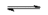 $H_2$
$Pb^{2+}+2e^-$
$H_2$
$Pb^{2+}+2e^-$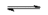 $Pb$
$Sn^{2+}+2e^-$
$Pb$
$Sn^{2+}+2e^-$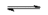 $Sn$
$Fe^{2+}+2e^-$
$Sn$
$Fe^{2+}+2e^-$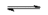 $Fe$
$Zn^{2+}+2e^-$
$Fe$
$Zn^{2+}+2e^-$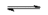 $Zn$
$Al^{3+}+3e^-$
$Zn$
$Al^{3+}+3e^-$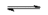 $Al$
$Mg^{2+}+2e^-$
$Al$
$Mg^{2+}+2e^-$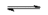 $Mg$
$Na^{+}+e^-$
$Mg$
$Na^{+}+e^-$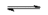 $Na$
$Ca^{2+}+2e^-$
$Na$
$Ca^{2+}+2e^-$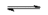 $Ca$
$K^{+}+e^-$
$Ca$
$K^{+}+e^-$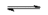 $K$
$Li^{+}+e^-$
$K$
$Li^{+}+e^-$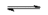 $Li$
$Li$
Marked in red: noble metals which do not react with $H^+$ Marked in blue: non noble metals which react with $H^+$

Zinc reacts with chlorhydric acid producing zinc chloride and hydrogen gas
$2H^{+}+2e^-\longrightarrow H_2(g$)
$Zn-2e^-\longrightarrow Zn^{2+}$
 $Zn +2H^+\longrightarrow Zn^{2+}+H_2(g)$
$Zn +2H^+\longrightarrow Zn^{2+}+H_2(g)$
Rain water contains $H^+$ ions coming from several acids, especially $H_2CO_3$:
So non noble metals will be attacked by rain water in nature and cannot be found there in elementary form.
On the other side $H^+$ cannot oxidise noble metals:
$Au, Hg, Ag, Cu$.
These metals can be found in their native states:
 Native (metallic) gold
Native (metallic) gold
 Native (metallic) silver
Native (metallic) silver
 Native (metallic) copper
Native (metallic) copper