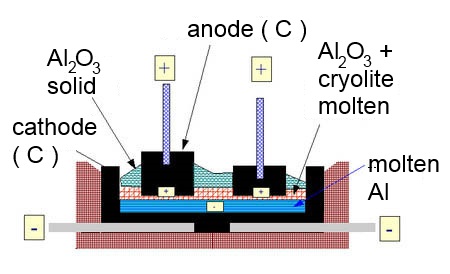
Electrolysis of bauxite $Al_2O_3$ in a molten bath (without water!) between graphite electrodes. Give the formulas of the ions which are in presence !
Ions: $Al^{3+}$ ; $O^{2-}$ from the melting of bauxite.
Look for the strongest oxidant - for the strongest reductant !
$Al^{3+}$ is the strongest oxidant $O^{2-}$ is the strongest reductant.
Write the equations of the reactions occuring at the electrodes !
Cathode: $Al^{3+}$ $+$ $3e^-$ $\longrightarrow$ $Al$ $2H^{+}$ $+$ $2e^-$ $\longrightarrow$ $H_2$ (reduction) Anode: $2O^{2-}$ $-$ $4e^-$ $\longrightarrow$ $O_2$ (which reacts with the $C$ of the electrode producing $CO_2$) (oxidation
This is the industrial process for the fabrication of aluminium. In order to operate at reasonnable temperatures, 10% cryolite $NaAlF_3$ is added to the molten bath.