






Metallic lead cristals appear at the negative electrode: $Pb^{2+}$ $+$ $2e^-$ $\longrightarrow$ $Pb(s)$ Lead(II) ions are reduced by the electrons provided by the generator.
At the cathode (electrode $\ominus$): Reduction
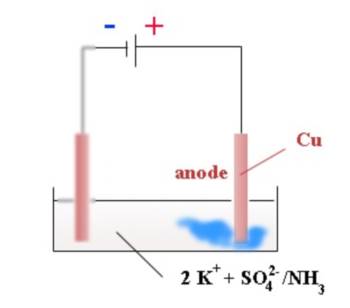
Copper(II) ions (complexed by ammonia) appear at the positive electrode : $Cu$ $-$ $2e^-$ $\longrightarrow $$Cu^{2+}$ Copper $Cu$ atoms of the anode are oxidised and loose electrons in favour of the generator.
At the anode (electrode $\oplus$): Oxidation
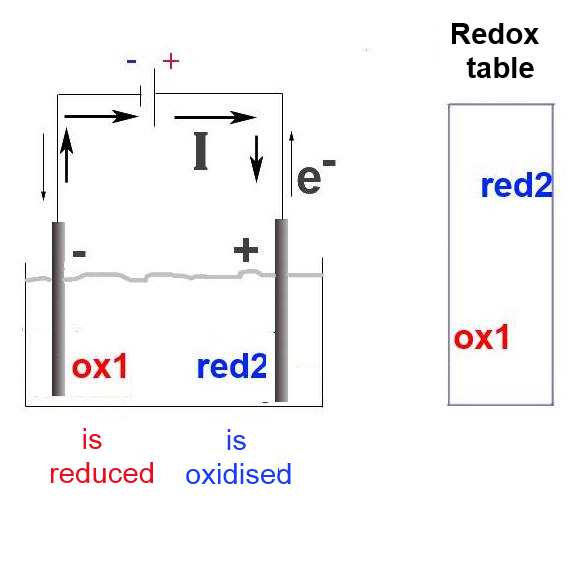
The oxidant 1 is not strong enough to tear spontaneously electrons from the reductant 2.
The generator is an electron pump. He enforces the exchange of electrons which would be impossible otherwise. At the cathode, the generator enforces the reduction of the strongest oxydant (found in solution or at the electrode) At the anode, the generator enforces the oxidation of the strongest reductant (found in solution or at the electrode ) In solution, the current is ensured by the migration of positive ions to the anode and negative ions to the cathode.

Part of the water molecules are always dissociated to hydrogen ions and hydroxide ions: $H_2O\longrightarrow H^++OH^-$
That is a very weak dissociation (one molecule out of 10000000 ! approximately), but it is important enough to consider $H^+$ and $OH^-$ in the forecast of an electrolysis in aqueos solution!
In our case above, we must consider the following species: $K^+$ $I^-$ $H^+$ $OH^-$ $H_2O$ By consulting the redox table, we see that: - $H^+$ is the strongest oxidant. $H^+$ will be reduced at the cathode: $2H^+$ $+$ $2e^-$ $\longrightarrow$ $H_2(g)$ When hydrogen ions disappear, there will be an excess of hydroxyde ions and that is the reason why phenolphalein turns to pink. - $I^-$ is the strongest reductant. $I^-$ will be oxidised at the anode: $2I^-$ $\longrightarrow$$ I_2$ The brownish caracteristic colour of iodine appears there.