





The voltage series is the redox table showing the metals(reductants, reducing agents) and their usual cations (oxidants, oxidizing agents) including the couple ($H^+$/$H_2$)
| $Au^{3+}+3e^-$ |  | $Au$ |
| $Hg^{2+}+2e^-$ |  | $Hg$ |
| $Ag^{+}+e^-$ |  | $Ag$ |
| $Cu^{2+}+2e^-$ |  | $Cu$ |
| $2H^{+}+2e^-$ |  | $H_2$ |
| $Pb^{2+}+2e^-$ |  | $Pb$ |
| $Sn^{2+}+2e^-$ |  | $Sn$ |
| $Fe^{2+}+2e^-$ |  | $Fe$ |
| $Zn^{2+}+2e^-$ |  | $Zn$ |
| $Al^{3+}+3e^-$ |  | $Al$ |
| $Mg^{2+}+2e^-$ |  | $Mg$ |
| $Na^{+}+e^-$ |  | $Na$ |
| $Ca^{2+}+2e^-$ |  | $Ca$ |
| $K^{+}+e^-$ |  | $K$ |
| $Li^{+}+e^-$ |  | $Li$ |
Each oxidant (left) can react with each reductant (right) situated below
Iron and copper(II) cation:

An iron rod introduced in a solution of copper(II) sulfate becomes covered with a red copper layer:
$Cu^{2+}+2e^- \longrightarrow Cu$
$Fe-2e^- \longrightarrow Fe^{2+}$
______________________________
$Cu^{2+}+Fe \longrightarrow Cu + Fe^{2+}$
Copper and silver cation:
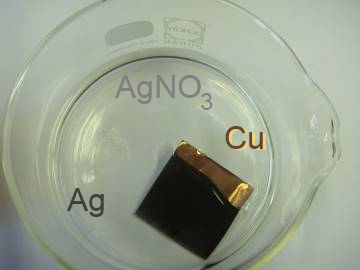
A copper sheet introduced in a silver nitrate solution becomes covered with a black silver layer:
$Ag^++e^-\longrightarrow Ag$
$Cu-2e^-\longrightarrow Cu^{2+}$
______________________________
$Cu +2Ag^+\longrightarrow Cu^{2+}+2Ag$
Zinc and lead nitrate:

Zinc pellets introduced in a lead nitrate solution becomes covered with black lead cristals:
$Pb^{2+}+2e^-\longrightarrow Pb$
$Zn-2e^-\longrightarrow Zn^{2+}$
______________________________
$Zn +Pb^{2+}\longrightarrow Zn^{2+}+Pb$