







Search:
The Lewis model is based on the octet rule and concerns simple molecules without transition elements, for instance:
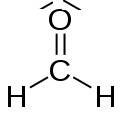 The Lewis model concerns also simple multiatomic ions, for example:
The Lewis model concerns also simple multiatomic ions, for example:
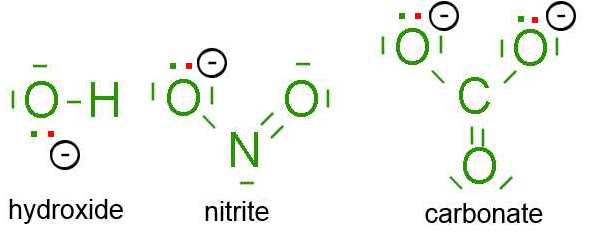 Green:
The outer electrons of the neutral atoms
Red:
The supplementary electrons which are responsible for the charge of the ion.
Green:
The outer electrons of the neutral atoms
Red:
The supplementary electrons which are responsible for the charge of the ion.
The atoms form as many covalent bonds as they have outer unpaired electrons

The oxygen atom O has to unpaired electrons able to form two covalent bonds in the water molecule, whereas the hydroxide ion has only a single unpaired electron left to form a covalent bond!