Search:
Taking or losing electrons, atoms tend often to adopt the structures $1s^2$ (first period) or $ns^2np^6$ (other periods) (octet rule). That leads us to the following elementary ions:
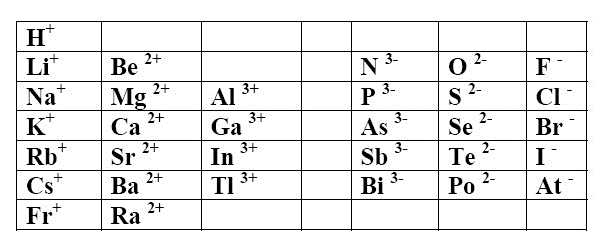
Positive simple ions (cations) adopt the name of the element
Examples $Li^+$ is the lithium ion (or cation) $Ca^{2+}$ is the calcium ion (or cation)
Negative simple ions (anions) take the suffix -ide Exceptions: $O^{2-}$ is the oxide ion $P^{3-}$ is the phosphide ion $N^{3-}$ is the nitride ion $S^{2-}$ is the sulfide ion
Examples $Cl^-$ is the chloride ion (or anion) $Se^{2-}$ is the selenide ion (or anion)
All the preceding ions are colourless.
Ions of transition elements don't follow the octet rule.
Sometimes they have a caracteristic color (which may be influenced by their surrounding):
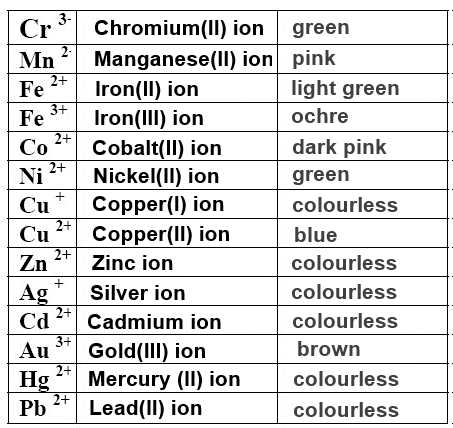

Cristal containing the $Cu^{2+}$ ion
Multiatomic ions have sometimes a caracteristic color (which may be influenced by their surrounding:
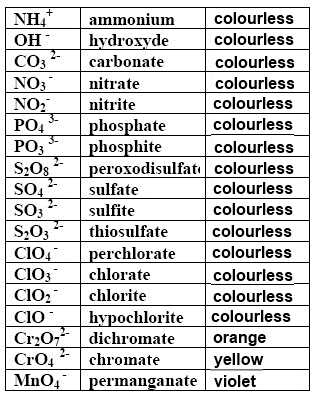

Cristals containing the $Cr_2O_7^{2-}$ ion
The addition of $H^+$ to an anion with multiple negative charge leads to an anion whose negative charge is diminished by one unit. Examples $HPO_4^{2-}$ is the (mono)hydrogen phosphate ion $H_2PO_4^{-}$ is the dihydrogen phosphate ion $HS^{-}$ is the hydrogen sulfide ion
Acid ions: Préfix: hydrogen Multiplicative factors : (mono), di tri (The prefix mono is optional)
The addition of $H^+$ to an anion with a simple negative charge leads no more to an ion, but to neutral molecule of a substance called acid $HClO_4$ is the perchloric acid $HClO_3$ is the chloric acid $HClO_2$ is the chlorous acid $HClO$ is the hypochlorous acid $HCl$ is the chlorhydric acid
Anion with suffix -ide → acid with suffix -hydric Anion with suffix -ite → acid with suffix -ous Anion with suffix -ate → acid with suffix -ic
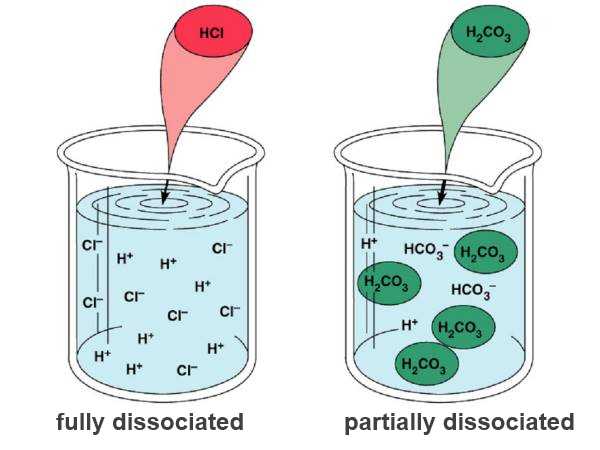
When acid molecules are introduced into water, they dissociate wholly or partially to form ions:
Examples:
$HCl$ 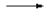 $H^+$ $+$ $Cl^-$
(complete dissociation: "strong" acid)
$H_2CO_3$
$H^+$ $+$ $Cl^-$
(complete dissociation: "strong" acid)
$H_2CO_3$ 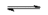 $H^+$ $+$ $HCO_3^-$
(partial dissociation: "weak " acid)
$HF$
$H^+$ $+$ $HCO_3^-$
(partial dissociation: "weak " acid)
$HF$ 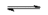 $H^+$ $+$ $F^-$
(partial dissociation: "weak " acid)
$H^+$ $+$ $F^-$
(partial dissociation: "weak " acid)