







Ammonia gas ( $NH_3$ ) releases in water the hydroxyde ion. Write the equation of this equilibrium.
$NH_3$ $+$ $H_2O$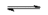 $ NH_4^+$ $+$ $OH^-$
$ NH_4^+$ $+$ $OH^-$
In water, what state is the copper(II) ion in?
$[Cu(H_2O)_5]^{2+}$
In tubes 2 et 3 a precipitate appears. Which one?
$Cu^{2+}(OH^-)_2$ , copper(II) hydroxide
Write the equation of this precipitation taking account of the real initial state of the copper(II) ion.
$[Cu(H_2O)_5]^{2+}$ $+2OH^-$ $\rightarrow$ $Cu^{2+}(OH^-)_2$ $+$ $5H_2O$
In the test tube 4 an dark blue colour appears. It is due to the complex ion tetramminecopper(II). Write the formula of that solution.
$[Cu(NH_3)_4]^{2+}$ $+$ $SO_4^{2-}$
Write the equation of the formation of this solution starting with the precipitate of test tubes 2 and 3.
$Cu^{2+}(OH^-)_2$ $+$ $4NH_3$ $\rightarrow$ $[Cu(NH_3)_4]^{2+}$ $+$ $2OH^-$