





The maximum quantity of silver chloride which dissolves in $1\;L$ of water at $25^oC$ is $1.27 \cdot 10^{-5}\;mol$ .
The .......... of $AgCl$ (not to be confused with the solubility product!!) is equal to $1.27 \cdot 10^{-5}\frac{mol}{L}$
The dissolution is immediately followed by a dissociation to ions.
$AgCl(s)$ 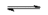 $Ag^+(aq)$ + $Cl^-(aq)$
$1\;L$ of a .......... solution of $AgCl$ in equilibrium with solid $AgCl$ contains .......... mol $Ag^+$ and .......... mol $Cl^-$.
The solubility product of [AgCl] at $25^oC$ is therefore equal to $K_s$ $=$ $[Ag^+][Cl^-]$ = .......... $\frac{mol^2}{L^2}$
$Ag^+(aq)$ + $Cl^-(aq)$
$1\;L$ of a .......... solution of $AgCl$ in equilibrium with solid $AgCl$ contains .......... mol $Ag^+$ and .......... mol $Cl^-$.
The solubility product of [AgCl] at $25^oC$ is therefore equal to $K_s$ $=$ $[Ag^+][Cl^-]$ = .......... $\frac{mol^2}{L^2}$
Click where you want to see the answer! Finish this question before beginning the next one!