





Give the definition of $K_s$ for the saturated solution resulting from the equilibrium (don't write (aq) ):
$Ag_3PO_4(s)$ 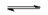 $3Ag^+(aq)$ $+$ $PO_4^{3-}(aq)$
$Ca_3(PO_4)_2(s)$
$3Ag^+(aq)$ $+$ $PO_4^{3-}(aq)$
$Ca_3(PO_4)_2(s)$ 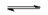 $3Ca^{2+}(aq)$ $+$ $2PO_4^{3-}(aq)$
$CaF_2(s)$
$3Ca^{2+}(aq)$ $+$ $2PO_4^{3-}(aq)$
$CaF_2(s)$ 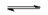 $Ca^{2+}(aq)$ $+$ $2F^-(aq)$
$Al_2S_3(s)$
$Ca^{2+}(aq)$ $+$ $2F^-(aq)$
$Al_2S_3(s)$ 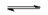 $2Al^{3+}(aq)$ $+$ $3S^{2-}(aq)$
$2Al^{3+}(aq)$ $+$ $3S^{2-}(aq)$