





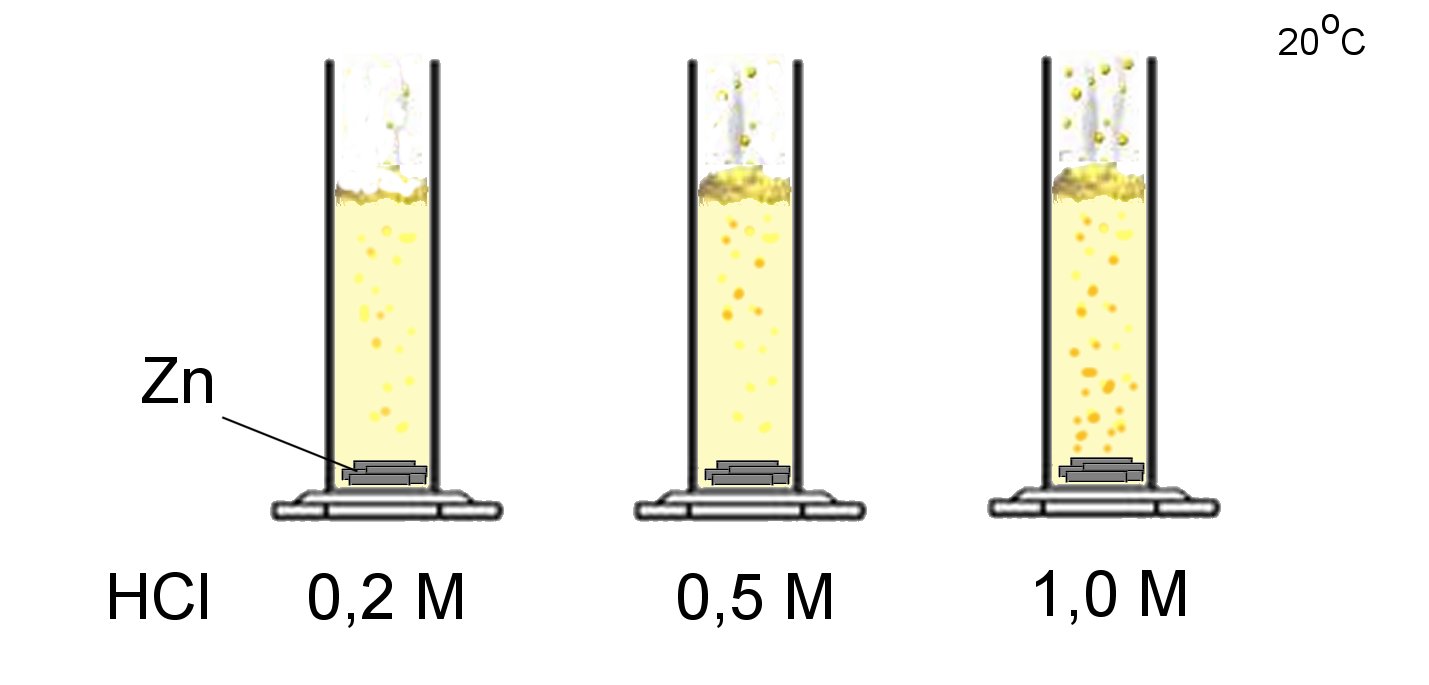
If the concentration of the reagents is high, the molecules are near to oneanother and they have more chance to meet:
Reaction rate increases with the concentration of reagents.
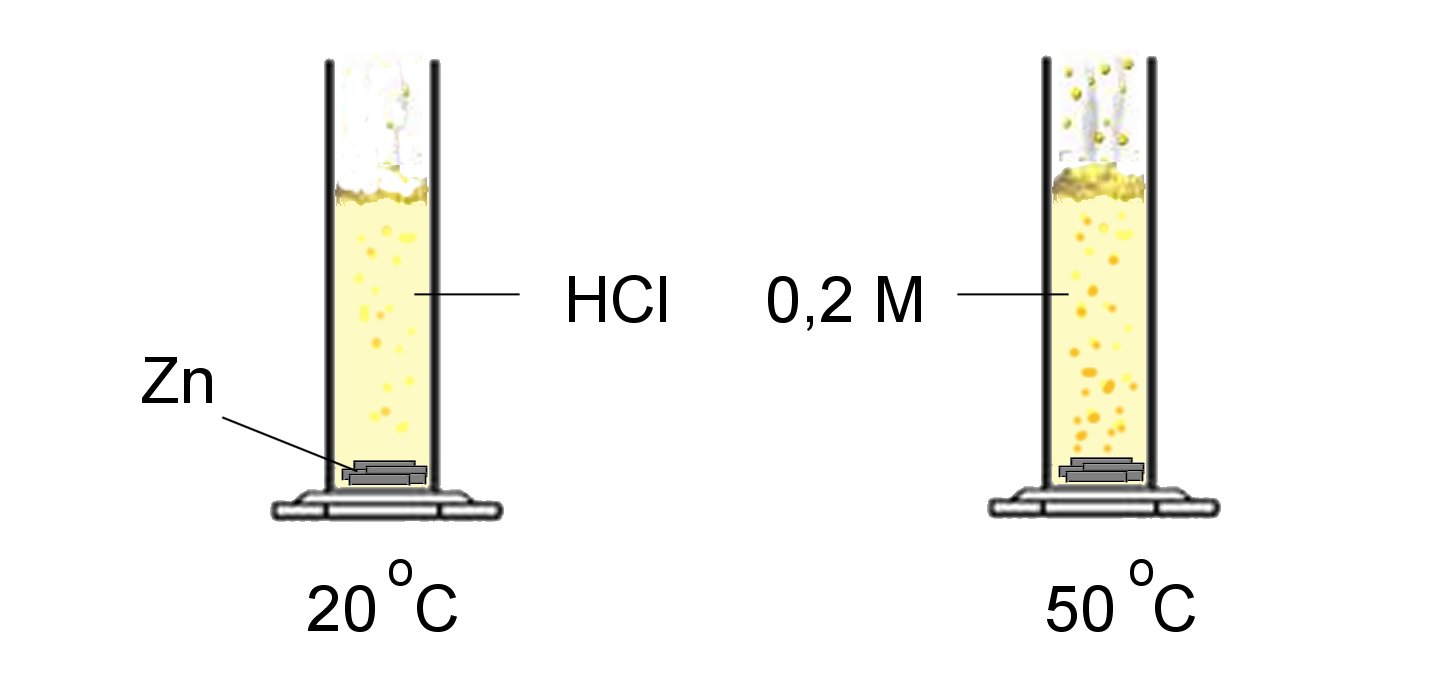
If the molecules are agitated they have more chance to meet:
Reaction rate increases with temperature.
Quite often the rate doubles as the temperature increases by $10^oC$.
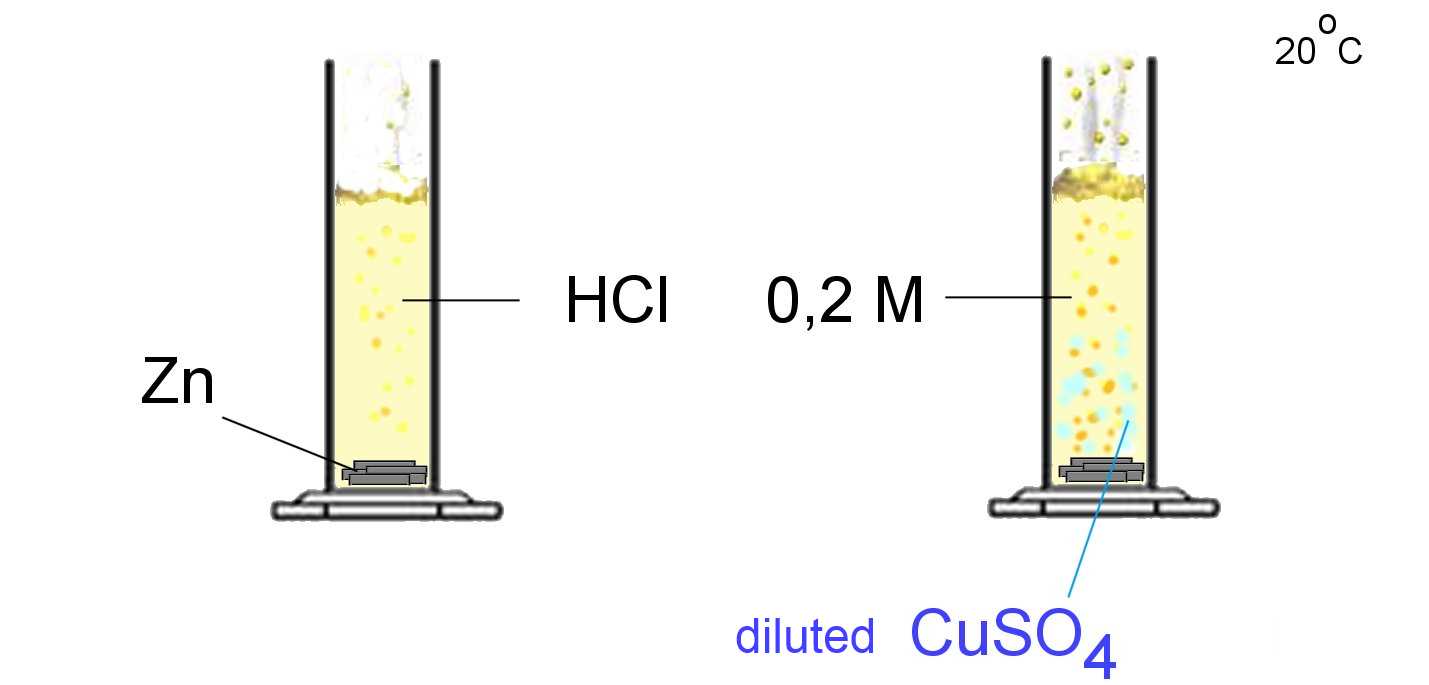
The catalyst for a reaction is a species which accelerates (or decelerates ) the reaction without participating as a reagent or a product.
$CuSO_4$ is a catalyst for the reaction: $Zn$ $+$ $2H^+$ $\longrightarrow$ $Zn^{2+}$ $+$ $H_2(g)$