





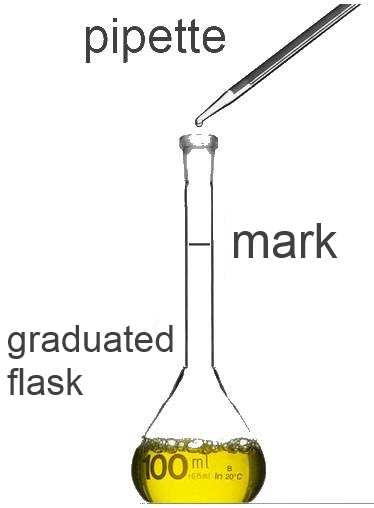
A concentrated solution is a solution with high concentration. A diluted solution is a solution with low concentration. Using a pipette a well defined volume of the concentrated solution is introduced in a graduated flask. The solvent is then introduced up to the mark, the flask is shaken. Finally we obtain a well defined volume of a diluted solution.
In the case of aqueous solutions (where the solvant is water), volumes may be added together:
V(diluted solution) = V(concentrated solution) + V(added water)
During dilution the number of moles of solute did not change:
n(solute in the diluted solution) = n(solute in the concentrated solution)