





The molar mass (M) of a substance is the mass (m) expressed in grams of one mole of molecules.
For instance, the mass of $6.023\cdot 10^{23}$ molecules of water is $18\;g$, therefore $ M_{H_2O}$ $=$ $18 \frac{g}{mol}$
The mass of a substance equals the number of moles multiplied by the mass of one mole( = molar mass)
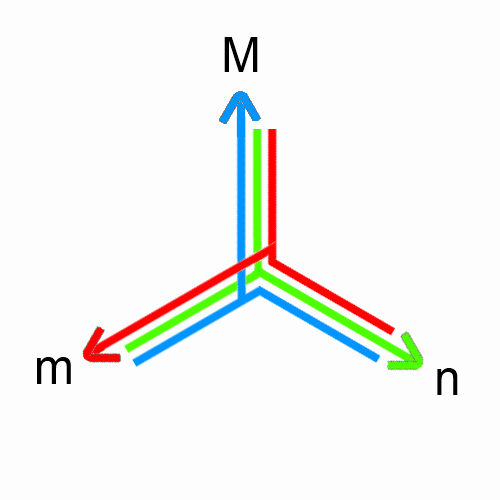
$m = n \cdot M$
For instance, the mass of 2 moles water is $m$ $=$ $2\cdot M_{H_2O}$ $=$ $2\cdot 18.02$ $=$ $36.04 g$
$n = \frac{m}{M}$
For instance, 36.04 g water are $n$ $=$ $\frac{36.04}{M_{H_2O}}$ $=$ $2 mol$
$M = \frac{m}{N}$
For instance, knowing that 36.04 g water are 2 mol , the molar mass of water is $M_{H_2O}$ $=$ $\frac{36.04}{2}$ $=$ $18.02 \frac{g}{mol}$