 → Back to the complete list of formulas
→ Back to the complete list of formulas
 → Back to the complete list of formulas
→ Back to the complete list of formulas
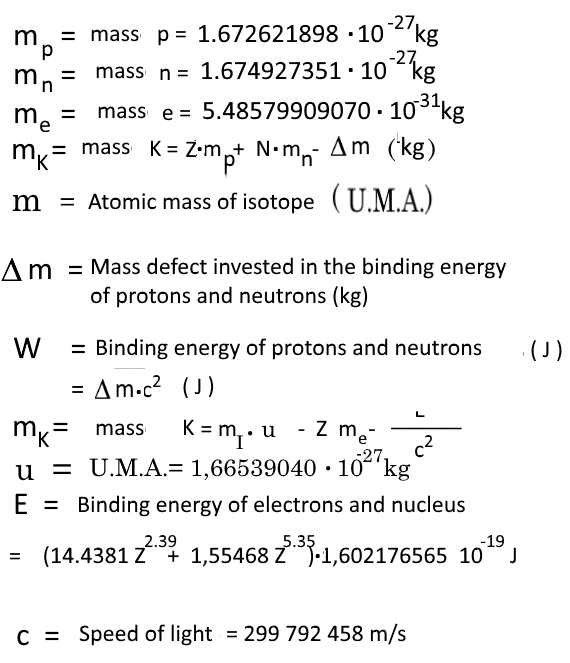
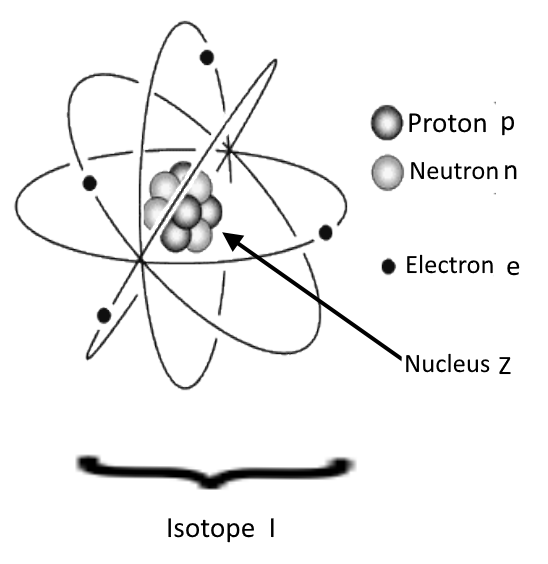
→ Atomic masses of the isotopes
→ Nuclear properties
Attention: 1 Mev = $1.602176565\cdot 10^{-13} \, J$;
The present page works with Joules!
If you want to introduce for example $1,6\cdot 10^{-9}$ type: 1.6E-9