

Reactions of alcohols with sodium

Reactions of alcohols with sodium
Equation
$R-OH+Na$ $\rightarrow$ $ RO^-Na^+ +\frac{1}{2}H_{2}$
A sodium alcoholate (alkoxide) is formed
The alcoholate ion is a strong base :
$RO^-+H_2O \rightarrow ROH+OH^-$
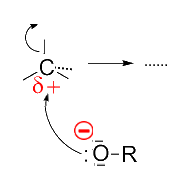
The alcoholate ion is a strong nucleophile
$ H_{2}$ is a gas !
One mole alcohol produces $\frac{1}{2}$ mole $H_{2}$
Reminder : Ideal gas law
NTP:
$n=\frac{V}{22,4}$
where $V$ is expressed in L
else:
$pV$ $=$ $nRT$
where
$V$ is expressed in $L$
$T$ is expressed in $K$
$P$ is expressed in $atm$
$R=0.083\frac{L\cdot atm}{mol\cdot K}$
Find how many moles of Hydrogen are formed by the reaction of $ 6.4\;g $ of methanol with excess sodium!
First look for the number of moles of methanol !
For answers, use (possibly multiple times) the arrows ↑ and ↓ at the top!
Complete please this question before moving on to the next!
$n_{CH_3OH}$ $=$ $\frac{m}{M}$ $=$ $\frac{6,4}{32}$ $=$ $0.2\; mol$
Then the number of moles of hydrogen !
One mole (mono)alcohol produces $\frac{1}{2}$ molHydrogen, so:
$n_{H_2}$ $=$ $\frac{0,2}{2}$ $=$ $0.1\; mol$


