Acid-base titration curves
Tutorial 1


 The acid / base titration curves
The acid / base titration curves
The titration curves describe the evolution of the pH as a function of the volume $ v $ of the added titrating substance.
If $ (v = V_e) $ (equivalence point), the initial number of moles of the titled substance is equal to the number of moles of the titrant added.
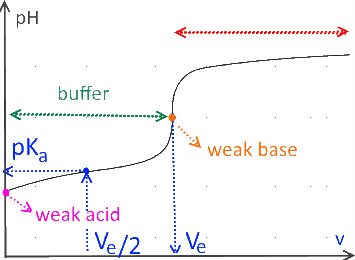
In the case of titration of weak acids or bases, the $ pH $ at the half equivalence point is equal to the $ pK_a $ of the titrated substance.
Strong acid titrated by a strong base
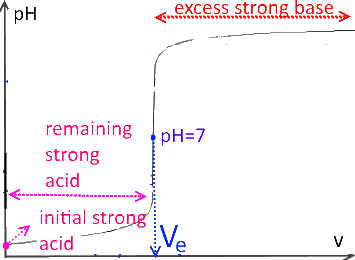
Strong base titrated by strong acid

Weak base titrated by strong acid

Weak acid titrated by a strong base
Here is a titration curve of an acid per $ NaOH\; 0.1\; M $ recorded using a pH meter:
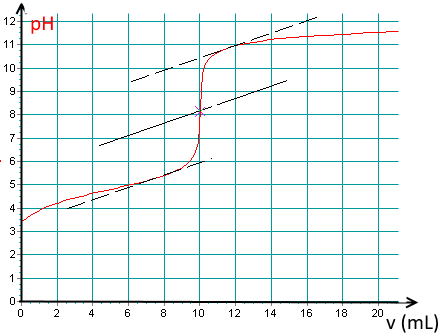
What kind of acid is it ?
For answers, use (possibly several times) the arrows ↑ Down! and ↓ Up!
Complete please this question before moving on to the next one!
It is a weak acid.
Determine the base volume added at the equivalence point!
(Notice the tangent method to find the E.P. pixel accurately!).
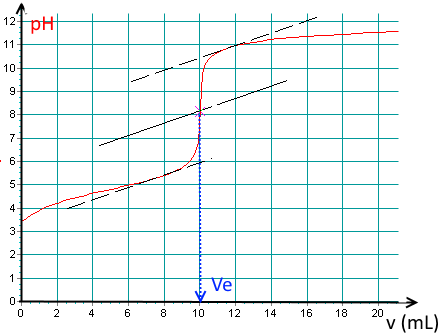
$V_e\approx 10.0\;mL$.
Find the $pK_a$ of the acid !
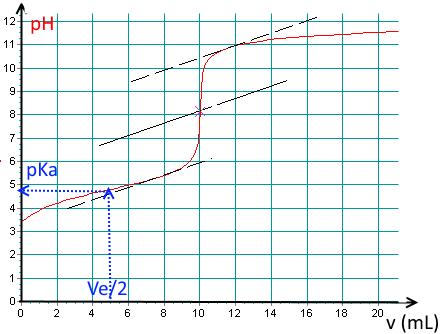
$pK_a\approx 4.8$.






