




Search:
Let $x$ $\frac{mol}{L}$ be the number of moles of calcium fluoride dissolved in $1$ L of a $0.01$ M solution of $NaF$ at $20^oC$.
$x$ is the "solubility" of calcium fluoride in this solution.
The dissolution of calcium fluoride
$CaF_2(s)$ 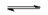 $Ca^{2+}(aq)$ + $2F^-(aq)$
$K_s=1.7\cdot 10^{-10}\frac{mol}{L}$
supplies per L .......... mol $Ca^{2+}$ and .......... mol $F^-$
Sodium fluoride contributes per liter with .......... mol $F^-$ , in such a way that there is finally in the saturated solution:
$[Ca^{2+}]$ = ..........$\frac{mol}{L}$
$[F^-]$ = ..........$\frac{mol}{L}$
The product of solubility at $25^oC $ of $CaF_2$, expressed as a function of $x$, is therefore worth $K_s=[Ca^{2+}][F^-]^2$ = .......... $\frac{mol^3}{L^3}$
- Establish the equation giving the solubility of calcium fluoride in $NaF$ $0.01$ M using these data!
- Solve with an efficient computing machine
$Ca^{2+}(aq)$ + $2F^-(aq)$
$K_s=1.7\cdot 10^{-10}\frac{mol}{L}$
supplies per L .......... mol $Ca^{2+}$ and .......... mol $F^-$
Sodium fluoride contributes per liter with .......... mol $F^-$ , in such a way that there is finally in the saturated solution:
$[Ca^{2+}]$ = ..........$\frac{mol}{L}$
$[F^-]$ = ..........$\frac{mol}{L}$
The product of solubility at $25^oC $ of $CaF_2$, expressed as a function of $x$, is therefore worth $K_s=[Ca^{2+}][F^-]^2$ = .......... $\frac{mol^3}{L^3}$
- Establish the equation giving the solubility of calcium fluoride in $NaF$ $0.01$ M using these data!
- Solve with an efficient computing machine
Calculation: ....... ....... .......
Click in the right order at the places where you would like to see the answer. Finish s.v.pl. This question before moving on to the next!