




Search:
At $250^oC$, phosphorus pentachloride is dissociated:
$PCl_5(g)$ 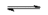 $PCl_3(g)+Cl_2(g)$
Each mole which dissociates produces .......... mole(s) $PCl_3$ and .......... mole(s) $Cl_2$
$PCl_3(g)+Cl_2(g)$
Each mole which dissociates produces .......... mole(s) $PCl_3$ and .......... mole(s) $Cl_2$
Click where you want to see the answer ! Finish please this exercise before beginning the next one!